Driving Innovation and Safety in Medical Devices
QNX delivers foundational software platforms that empower the medical device industry to create the next generation of life-critical applications. From complex surgical robotics and precise diagnostic equipment to reliable patient monitoring and drug delivery systems, our safety-certified and secure solutions are engineered to meet the stringent demands of modern healthcare.
We enable manufacturers to build cutting-edge medical devices with the confidence that they are built on a bedrock of proven reliability and security, ultimately contributing to improved patient outcomes and the advancement of medical technology.









Deliver Secure, Safety-Certified Medical Devices
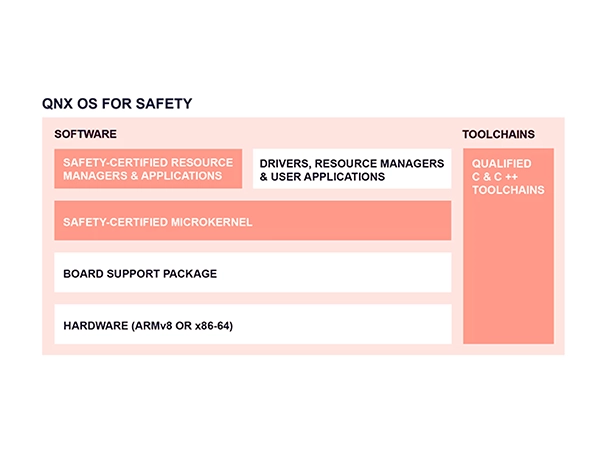
Whether you’re building a new product, a new prototype, or retooling an existing product, QNX® software is trusted by medical device manufacturers to support a broad range of life-critical and graphics-rich medical devices. Our foundational software is time-tested, inherently secure, and purpose-built for embedded systems. We pre-certified our QNX® OS for Safety and QNX® Hypervisor for Safety products to IEC 62304 Class C, and have launched QNX OS for Safety in the Cloud to help optimize your product lifecycle.
Download our Medical Solutions Guide to learn how QNX can help you with your medical applications.
How Healthcare Can Benefit from QNX
Precision Software at the Heart of Surgical Robotics
Robot-assisted procedures are revolutionizing patient care, cutting complications, speeding up recoveries, and shortening the length of hospital stays. In surgery, a single 100-millisecond hiccup can mean the difference between success and tragedy.
QNX OS 8.0 is a real-time operating system that processes interrupts in under 20 microseconds with minimal jitter. By slashing both latency and jitter, QNX buys precious milliseconds for complex surgical applications, assisting surgeons in making life-saving decisions in real time. Today, eight out of ten leading surgical robotics manufacturers build on QNX.
Foundational software that:
- Is safety-certified and has a secure microkernel architecture that is trusted by leading medical device manufacturers.
- Is standards-based and works across safety and non-safety related systems and is scalable across medical device products and product lines and families.
- Has adaptive partitioning and prioritization scheduling to help ensure critical processes get the cycles they need to complete tasks on time.
- Enables granular security profiles and the ability to monitor and audit for system integrity.
Keep Your Medical Device Safe and Secure
Build with standards-compliant foundational software that can help accelerate certification of IEC 62304 up to Class C.


Ensure Reliability



Strengthen Cybersecurity



Reduce Costs

Cybersecurity for Medical Devices: Building Standards-Compliant Software Solutions

Need More Information?
Download “5 Criteria to Consider When Choosing a Medical Device OS” to learn about:
1. Safety certification
2. Code traceability and software of unknown provenance (SOUP)
3. OS architectures
4. Cost and time-to-market
5. Long-term support and complete lifecycle licensing
Medical Webinar Series



Shortening Medical Software Development



The Importance of Strong Cybersecurity Principles


Streamlining Safety Certifications in Medical Devices



Machine Learning in Medical Devices
Meet with QNX Functional Safety Experts and Get Help with Your Next Certification or Safety-Critical Project.
Related Solutions


QNX OS for Safety



QNX Hypervisor for Safety



Professional Services
Additional Resources



FAQs about QNX for Medical Device Software
Which QNX products are safety-certified to IEC 62304 standard?
The QNX OS for Safety and QNX Hypervisor for Safety are certified to IEC 62304 Class C. QNX® Hypervisor for Safety offers the same trusted functionality and performance as QNX® OS 8.0 plus virtualization support. These products are safety-certified variants of QNX OS 8.0 and the QNX Hypervisor, respectively.
What are the benefits of a safety-certified RTOS?
Your safety certification process is susceptible to countless deviations. From integration of effective lines of code determined by functional requirements to analysis of vulnerabilities and meeting certification criteria and standards, there are numerous factors that could influence your go-to-market timeline and associated expenses with achieving a safety-certified medical device. Building with a safety certified RTOS eases these processes, allowing you to certify only the components you develop. More benefits of a safety-certified RTOS such as the QNX OS for Safety include:
- Reduced development effort. QNX-documented safety recommendations and restrictions reduce the time and effort (e.g., testing, analysis, documentation) needed for you to develop these materials from scratch—not to mention ensures you design a safe system using these guidelines
- Accelerated time-to-market. The development process can be streamlined to leverage QNX safety artifacts for quicker approvals from regulatory bodies including Class I, II, or III medical devices.
- Maintenance and support. QNX products are maintained and supported throughout the product lifecycle with the utmost rigger, satisfying the medical device update policies required by regulatory bodies and reducing any rework that might be required with regulatory bodies.
- Cost savings. The cost of purchasing safety certification documents can be reduced as it relates to documentation development time and testing efforts. This cost is amplified if there are product updates that require resubmission of documentation.
What advantages does the microkernel architecture offer?
At the heart of QNX technologies is the microkernel based QNX OS 8.0. The microkernel architecture minimizes downtime, is safe by design, and reduces cyberattack surfaces through isolation and separation mechanisms. Device drivers, Board support packages, and system services run alongside applications, separated from one another and outside kernel space. Running all OS services outside of kernel space enables highly available, fault-tolerant designs—the failure of one application or service will not crash the kernel or other services or applications.
Building on QNX OS 8.0 can help you to develop more resilient and reliable systems.
How does the RTOS help strengthen cybersecurity for medical devices?
The frequency of attacks on medical devices and the healthcare sector is rapidly increasing, necessitating proactive measures to ensure connected devices are designed and developed using secure products. Strengthening cybersecurity requires a proactive approach, which includes designing robust secure system architectures, code, and hardware. It is the baseline for integrating security techniques and technologies into medical devices.
QNX solutions provide a layered approach to security that won’t hamper functionality. QNX OS 8.0 comes with a portfolio of security features and reduces attack surfaces by running services outside of the kernel space and provides granular control of system privilege levels, secure boot, and an AES-256 encrypted and self-verifying filesystem.
With the new FDA Cybersecurity requirements and the IMDRF Cybersecurity Guidelines, it’s imperative to build cybersecurity standards-compliant software solutions for medical devices.
How easy is it to develop with QNX OS 8.0?
QNX® Software Development Platform (SDP) 8.0 is a comprehensive software platform for building medical devices. It includes the latest QNX OS 8.0, QNX® Momentics® Tool Suite and QNX Software Center to provide a full-featured, microkernel RTOS for ARM and x86 platforms with 64-bit support. It also includes a High-Performance Networking stack to support basic-to-complex networking requirements. Its comprehensive POSIX-compliant development environment will be familiar to anyone who’s worked with Linux®.
What are the real-time performance advantages of QNX RTOS for medical devices?
The microkernel architecture of the QNX RTOS ensures reliable and predictable response times—critical for patient safety in real-time applications. With deterministic scheduling and resource management, QNX can provide consistent processing, reducing latency in vital functions, including:
- Data monitoring (e.g. accurate readings of patient NMT, entropy, EEG, anesthetic-agent measurements, volumetric CO2 and O2, PiCCO, cardiac output, SvO2 and dual SpO2 levels)
- Diagnostics (e.g. precision processing of assays or analyzers to reduce loss of samples from errors and restarts)
- Device control (e.g. low jitter responses to maneuver surgical robotic arms)
This real-time capability helps medical devices maintain precise timing, accuracy, and reliability (fault-tolerant)—all essential for delivering effective healthcare solutions.